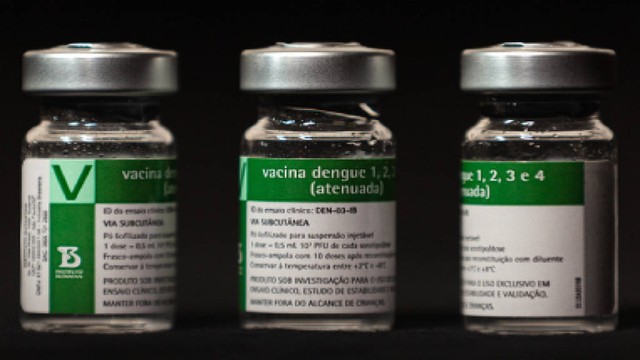
São Paulo, Nov 27 (V7N) – Brazil has become the first country in the world to approve a single-dose dengue vaccine. Developed by the Butantan Institute in São Paulo, the vaccine, named “Butantan-DV,” received regulatory approval on Wednesday, November 26, in what Brazilian authorities have called a “historic achievement.” The rollout comes as dengue cases are rising in many countries due to increasing temperatures.
Brazil’s health regulatory agency, Anvisa, has approved the vaccine for individuals aged 12 to 59. Previously, the only available dengue vaccine, TAK-003, required two doses spaced three months apart. Following eight months of nationwide trials, the single-dose Butantan-DV vaccine has now been cleared, allowing faster and easier immunization across the country.
Speaking at a press conference in São Paulo, Butantan Institute Director Esper Calas described the approval as a landmark achievement for Brazil’s scientific and healthcare sectors. “For decades, this disease has caused havoc, and now we have a powerful tool to fight it,” he said.
Production of the vaccine will be supported by a partnership with the Chinese company UsibioLogics, which is expected to produce approximately three million doses by the second half of 2026.
Before approval, the vaccine was tested on 16,000 volunteers across Brazil, showing 91.6% efficacy against severe dengue infections.
Dengue is transmitted to humans through the Aedes aegypti mosquito. According to the World Health Organization, more than 146 million people have been infected with dengue globally to date, resulting in nearly 12,000 deaths.
This milestone positions Brazil at the forefront of dengue prevention, offering hope for reducing the burden of this mosquito-borne disease worldwide.
END/WD/SMA/




























Comment: